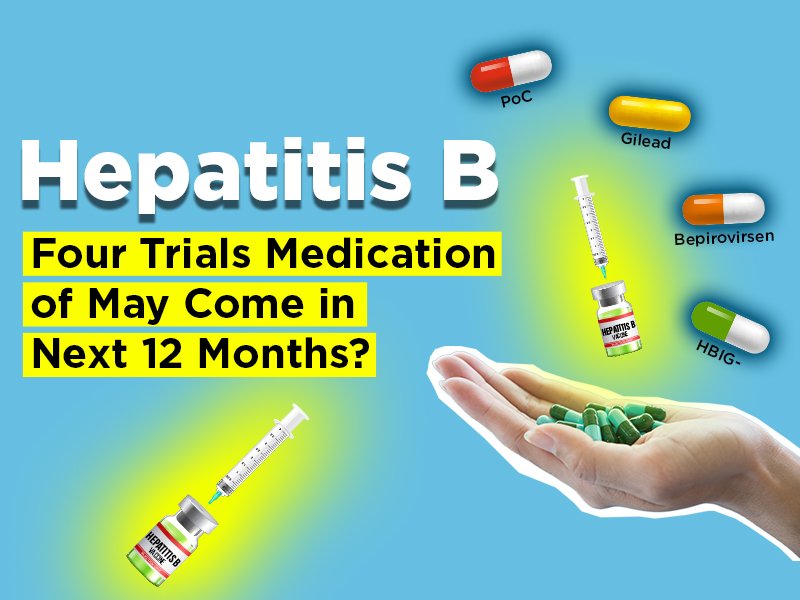
GSK developing a new product for Hepatitis B patients as “Bepirovirsen Sodium” according to GlobalData. Who is GSK? GSK, is mainly a British multinational pharmaceutical and biotechnology a global healthcare company. GSK focuses on developing, manufacturing, and commercializing general medicines, specialty medicines and vaccines.
In which Sectors GSK do Research?
GSK offers drugs for the treatment of diseases such as HBV, HIV, respiratory, cancer, immuno-inflammation, anti-viral, central nervous system (CNS), metabolic, cardiovascular, and urogenital, anti-bacterial, dermatology and rare diseases.
Bepirovirsen Sodium Phase II Trial
Bepirovirsen Sodium is under clinical development by GSK and currently in Phase II going on for Hepatitis B. What is the success rate of phase II trial? according to GlobalData, Phase II drugs for Hepatitis B have a 36% Phase transition success rate as of now. Bepirovirsen Mechanism of Action.
When Phase III Trial will Start?
GSK have the target indication benchmark for progressing into Phase III shortly.
What is Bepirovirsen Sodium
The product named as IONIS-HBVRx a combination of ISIS-HBVRx, ISIS-GSK3Rx is under development for treatment of chronic career patients with hepatitis B infection.
How Bepirovirsen Sodium Will Work?
It is directed through subcutaneous injection. The drug candidate targets viral HBV mRNA and reduce the production of viral proteins associated with HBV infection, replication, and targets TLR8. Please watch this video to know the details of Bepirovirsen Sodium.